Storing solar energy for long periods is not an easy task. Through photovoltaics, it is possible to transform it into electricity that could be stored for days in the batteries. But what if we wanted to store heat? Well, this issue could become complicated.
Fortunately, nowadays chemistry represents and proposes solutions. Research dedicated to solar gain allows studying materials that may act as a sort of battery thanks to their chemical bonds.
Lancaster University research
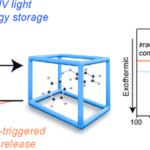
Researchers from Lancaster University analyzed a particular metal-organic structure or MOF which forms the basis of crystalline material. They found out that this structure is able to store energy at room temperature for months and release it on demand as heat.
Implementation of these materials could allow researchers to find another smart and innovative solution: harness solar energy during summer months and store it for winter use when there is less solar energy. This system could be exploited for different applications:
- heating in remote places
- as an ecological alternative to traditional heating in offices and homes.
- as a thin coating attached to the surface of buildings
- on the windscreens of cars where the stored heat could be useful for de-icing of the glass in freezing winter mornings
New MOF-Based Material
MOFs are made up of a network of metal ions connected by carbon-based molecules to create 3D structures. A key property of this class of compounds is porosity. This means that they can form composite materials by adding other small molecules inside their structures.
The research focused specifically on a metal-organic structure previously synthesized by a separate research team at the Kyoto University in Japan and known as “DMOF1“. Starting from this element, the scientists charged the pores of the MOF with azobenzene molecules. This compound absorbs light and acts as a molecular photo-switch. In other words, it can change its shape (isomerism) and its physical and chemical characteristics in response to an external stimulus, such as heat or light. This means that it can move from a “more energetic” to a “less energetic” form.
During the experiments, the researchers exposed the material to UV light. The rays led the azobenzene molecules to change shape to a strained configuration within the MOF pores. This process stores the sun’s energy in a manner similar to the potential energy of a bent spring. In order to release it, it is necessary to apply just a little external heat. This works as a trigger for a quick change of state and in turn, it provides more heat.
What said Dr John Griffin, Senior Lecturer in Materials Chemistry, Lancaster University
“The material functions a bit like phase change materials, which are used to supply heat in hand warmers. However, while hand warmers need to be heated in order to recharge them, the nice thing about this material is that it captures ‘free’ energy directly from the sun. It also has no moving or electronic parts and so there are no losses involved in the storage and release of the solar energy. We hope that with further development we will be able to make other materials which store even more energy”
Additional tests revealed the material could store the energy for a minimum of four months. The longer duration of the stored energy will make it potential cross-seasonal storage.
If you want to stay updated on news, technologies and activities, subscribe to Compositi Newsletter
Source: Lancaster University